Nancy A. Speck, Ph.D.
About PI
Nancy A. Speck, Ph.D.
John W. Eckman Professor in Medical Science II
Chair and Professor of Cell and Developmental Biology
Investigator, Abramson Family Cancer Research Institute
Member, Abramson Cancer Center
Member, Institute for Regenerative Medicine
Co-Leader, Hematologic Malignancies Program, Abramson Cancer Center
Co-Director, Hematopoietic Stem Cell Program, Institute of Regenerative Medicine
Department: Cell and Developmental Biology
Research Interests
Hematopoietic stem cells and leukemia using mouse models
Key Words
Hematopoietic stem cells, leukemia, core binding factors, transcription, inherited leukemia predisposition
Research Subjects
The work in my laboratory is centered on the core binding factor (Runx1-CBFβ) and its roles in hematopoietic stem cell (HSC) formation and function. We study how HSCs form in the embryo, the step at which HSC formation is dependent on Runx1-CBFβ, the biochemical functions of Runx1-CBFβ, and how mutations in the genes encoding Runx1-CBFβ generate pre-leukemic stem cells.
I. Hematopoietic stem cell formation in the embryo
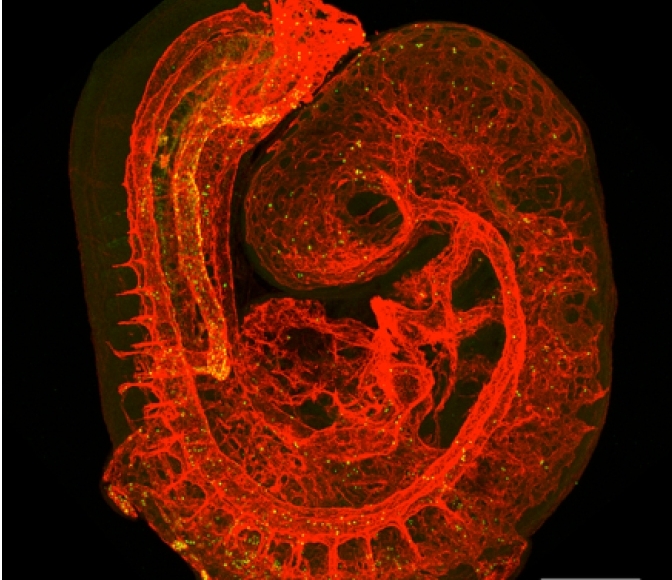
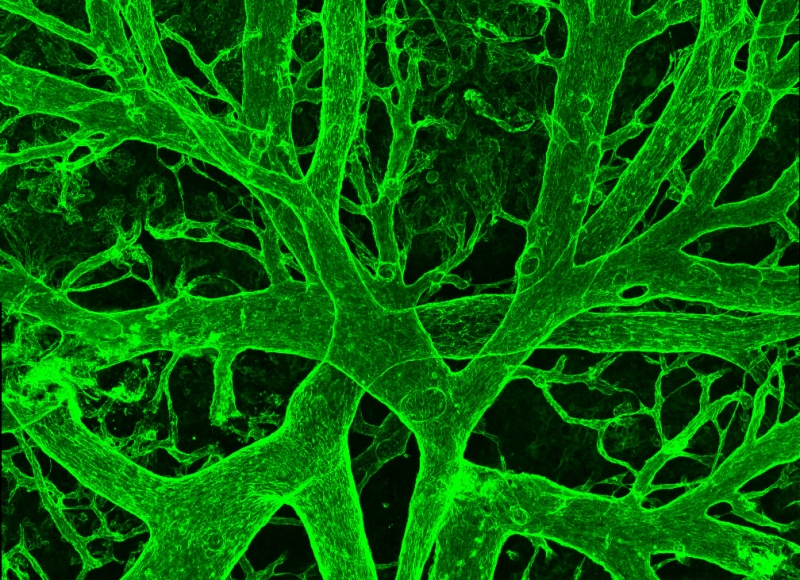
A major focus of my laboratory is to understand how hematopoietic stem cells (HSCs) form during normal embryonic development. HSCs and the earlier populations of committed progenitors differentiate from a small, transient population of endothelial cells referred to as “hemogenic endothelium”. We demonstrated that the transcription factor Runx1 was a specific marker of hemogenic endothelium, and that Runx1 is absolutely required for the formation of blood from hemogenic endothelium (North et al. Development, 1999). We helped settle an almost 100 year debate over whether hemogenic endothelium actually exists and demonstrated that at least 95% of all adult HSCs are derived from hemogenic endothelium in the embryo (Chen et al. Nature 2009). Another important discovery was that hemogenic endothelium is functionally heterogeneous – some hemogenic endothelial cells produce committed hematopoietic progenitors and some produce HSCs (Chen et al. Cell Stem Cell, 2011). We are currently trying to understand how the decision between producing a committed progenitor and multi-lineage repopulating HSC is made (Zhu et al. Blood, 2020).
II. Runx1 mutations in the pre-leukemic stem cell
Runx1 function is absolutely essential for the formation of HSCs in the embryo, but once HSCs colonize the fetal liver, they are no longer exquisitely Runx1 dependent (Chen et al. Nature 2009, Tober et al. Development, 2013). However, HSCs containing loss of function (LOF) Runx1 mutations are not normal – they are preleukemic stem cells (pre-LSCs) that are targets for secondary mutations. LOF RUNX1 mutations are common in multiple hematopoietic malignancies including acute myelogenous leukemia, acute lymphocytic leukemia, chronic myelomonocytic leukemia, and in the preleukemic myelodysplastic syndrome.
We are examining the functional consequences of Runx1 LOF mutations, both at a cellular and molecular level. An important discovery is that innate immune signaling is elevated in Runx1 mutant myeloid cells (Bellissimo, Blood Adv. 2020; Zezulin et al. Genes Dev. 2023). This results in the over-production of inflammatory cytokines and chemokines when innate immune cells (particularly neutrophils) are stimulated with bacterial ligands. We are examining whether the overproduction of inflammatory molecules by myeloid cells impacts the properties of HSCs, and how that elevates leukemia risk.